Autoimmune diseases happen when our immune system attacks our body instead of looking after it. There are a complex mosaic of causes, though we may be able to disentangle some of them.
Note: this is a big post for a big subject. If you have, or know anyone who has an autoimmune condition or indeed any other complex chronic illness – then it is worth reading. There is some really useful stuff in here!
Some of the most complex health problems are when the body’s immune system turns against other tissues in the body. Rheumatoid arthritis, Crohns, psoriasis, MS and similar diseases are familiar examples involving joints, gut, skin and nerves, respectively, but almost any tissue can be attacked and in some cases, like lupus, the whole body. Autoimmunity is also now implicated as a key factor in ME/Chronic Fatigue Syndrome, Long Covid, post-viral syndromes, Lyme disease and other disabling and mysterious conditions. These are among the outcomes of what looks like civil war! So the first question is how on earth does one pull the two sides apart?
The general medical solution is to suppress the relevant immune functions. This is still the most effective way to get relief. It is important to acknowledge how the fate of people suffering from rheumatism and other immune problems was transformed by the arrival of prescription steroids 70 years ago. Steroids like prednisolone, the non-steroidal anti-inflammatories (NSAIDs) like ibuprofen, and other strong immunosuppressants such as azathioprene, methotrexate, hydroxychloroquine, and cyclosporine can make life tolerable for sufferers, even though side-effects are common and sometimes serious. The new generation of ‘biologics’ more selectively block specific parts of the immune system and inflammatory processes, though are more complex (ie. cost much more), are harder to administer and are also prone to adverse effects.
Is it possible to find another way to reduce the pain and damage of autoimmunity? The conventional medical view is that these diseases are not curable – that by the time symptoms appear the immune system is locked in on the target tissue and cannot be reprogrammed, only suppressed or blocked. However, recent research discoveries have shown that some of the drivers of immune damage are able to be corrected: the symptoms are not set in stone!
So another question could be how good does the condition get on its own? Most autoimmune conditions come with good days and bad. Some are notably variable and may be marked by early fleeting symptoms that come and go. What could be going better on the good days? Is it possible to help the body have more of them?
The processes of auto-immunity are indeed highly complex, but that fact also provides multiple opportunities to modulate them. To do this, it is useful to pick out the key elements.
(Note: If you need to check back through the articles in our Introduction to Immunity to help follow these arguments.)
A widely accepted interpretation is that auto-immune problems are exaggerated immune defences. The immune cells, like B-cells, T-cells, natural killer cells and their associated antibodies and inflammatory mediators are doing their job, but either too well or because they have been misinformed. Can we find ways to cut back on those influences driving the immune system?
Let us look at some of the pathways to an autoimmune disease.
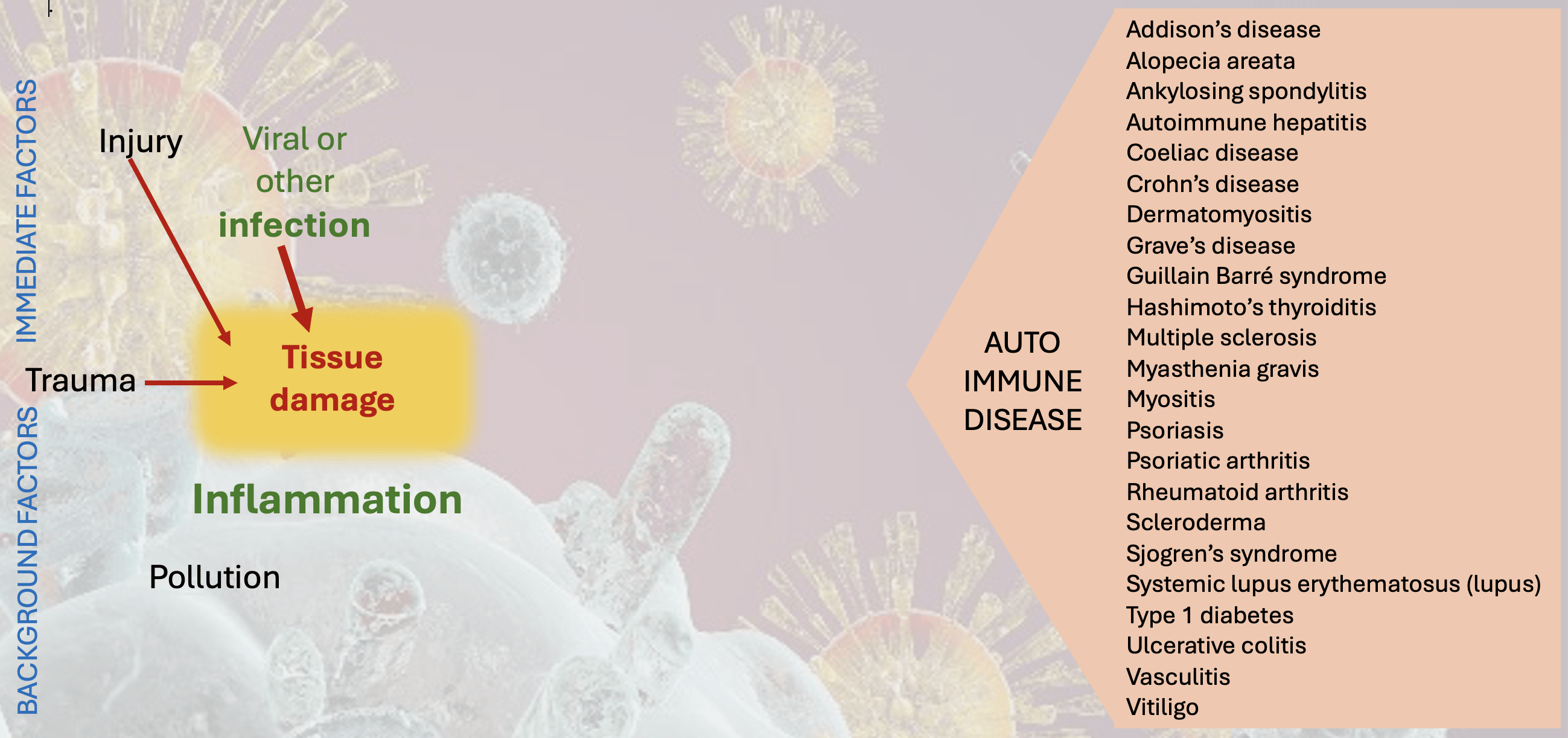
There are typically two stages in the path towards autoimmune disease. Let’s use the infographics above and below to walk through them. Note that all the following steps can take years or even decades to unfold. For example autoantibodies have been detected well before disease and as indicated in other posts may not predict it (eg rheumatoid factor – RF – is an autoantibody found in 5% of the population but rheumatoid arthritis itself affects 1%).
The first step is traumatic tissue damage. This can occur with any injury, poisoning (eg with heavy metals, DDT, PCBs), radiation, or circulatory failure (ischaemia) but is particularly linked with infections. What makes such trauma dangerous is when it is accompanied by the release of ‘autoantigens‘. These may be the healthy contents from inside our cells, normally kept away from the immune system outside the cell walls. When cells die normally (apoptosis) they are tidily disposed of by intracellular digestion and then by those very efficient innate immune cells. When damage is traumatic these cleaning agents may be overwhelmed with material and cell contents could flood into the body fluids, these then ‘recognised’ as antigens by the antibody-producing adaptive immune system. ‘Necrosis‘ is the classic autoantigen release event but any major trauma can do it. Bacterial infections are also liable to induce this scale of destruction and are now linked to autoimmune diseases include E.coli, Streptococcus, Kelbsiella, Staphylococcus aureus and Proteus. Borrelia burgdorfei the spirochete pathogen in Lyme’s disease, has also been implicated as a possible provocation.
Another source of autoantigens is when a cell is infected with viruses and generates new viral antigens which then become associated with that tissue. Viruses often associated with kicking off long term immunity problems include Epstein-Barr virus (EBV – causing glandular fever), rubella, herpes, varicella, coxsackie, HIV and … Covid 19.
One consequence of necrotic or other tissue injury is the generation of danger signals. A technical name for these is DAMPs (Damage Associated Molecular Patterns), molecules that are released by damaged or dying cells that activate the innate immune system. DAMPs start as good things: they normally prompt regeneration of the tissues, but in excess are major initiators of inflammation and disrupted immunity.
An important cofactor in these early stages of immune provocation is local inflammation, initially another cleansing function that can also outstay its welcome, with the generation of yet more damaging cytokines.
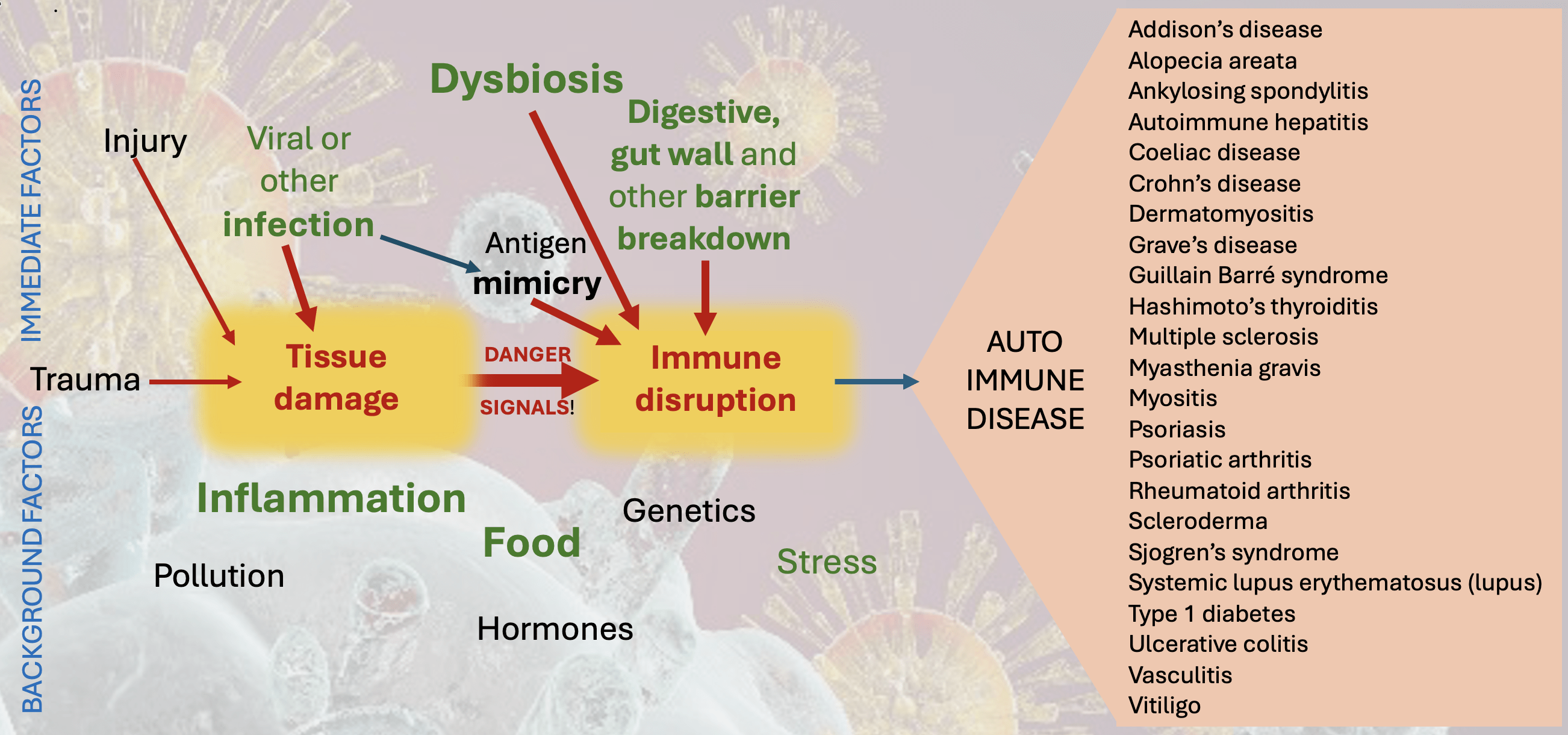
The second key stage is an immune disruption. This most often follows tissue damage, although it can occur spontaneously. Normal immune processes that should be directed to tidy up foreign proteins are re-programmed to attack own cells and tissues. Antibody-producing B cells and active T cells both recognise auto-antigens, a process which becomes amplified when they also activate innate immune cells. This triggers a chronic inflammatory process that disrupts normal tissue function. Various other moderating factors (types of T-cell for example) can be further disrupted, and inflamed innate immune cells can further disturb checks and balances.
More is now understood about factors that can drive this disruption. The first is molecular or antigen ‘mimicry‘. It has been discovered that sections of protein on some pathogens can be exactly the same as those on the outer wall of some host cells. One of the first discoveries was a sequence on the bacterium Klebsiella that is the same as on blood cells in people with a genetic type called HLA B27. People with this genotype are overwhelmingly more likey to suffer from the rheumatic-like condition ankylosing spondylitis. It is now accepted that in these individuals Klebsiella infections prompt immune responses that turn by mistake against their own tissues. There are now many more identified examples of this mimicry or ‘immunological cross reactivity’.
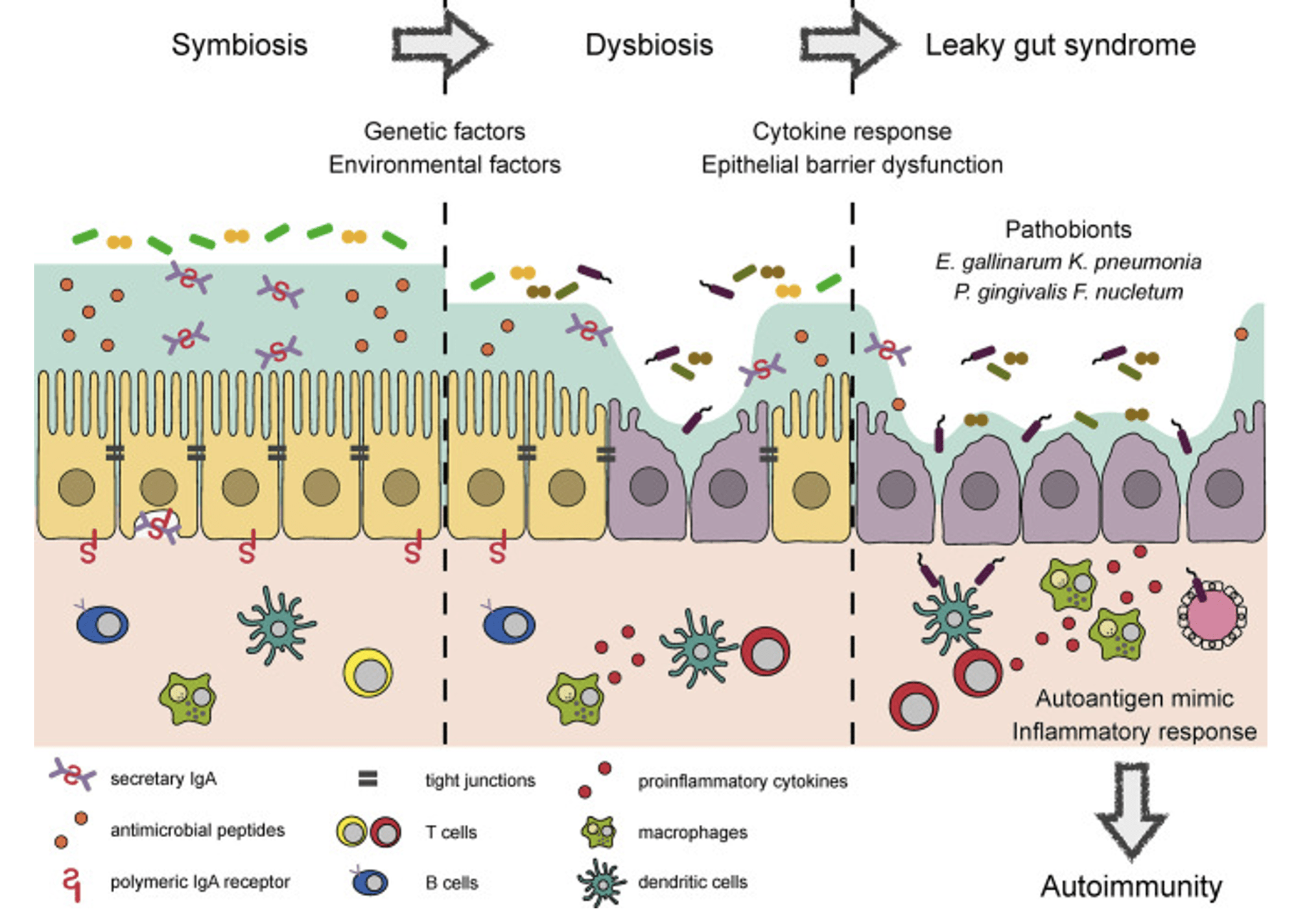
Other powerful influences on immune disruption include disturbed microbiomes (dysbiosis), particularly of the gut and mouth, but including in the lungs and skin. It seems that healthy microbiota really do keep the immune system in check. Antibiotic prescriptions and a diet too high in processed foods and refined carbohydrates thus may exacerbate immune disruption. Disturbances in the microbiome have inceasingly been implicated in many autoimmune diseases.
As with other immunological problems we have seen that a really important factor is the integrity of barrier defences. Breakdown in the protection provided by the gut wall (‘leaky gut’) is particularly implicated in chronic inflammatory and immunological disease, and issues can also arise in respiratory mucosa and skin. Almost all the major autoimmune diseases have now been linked to disturbances at the gut wall. This includes the prospect that bacteria, even friendly ones like Lactobacillus, can move into the tissues from the gut and induce autoimmune complications directly.
An inceasingly used marker of gut wall breakdown is the blood levels of zonulin, a protein analogous to that produced by cholera infection, High zonulin levels have been detected in a wide range of autoimmune conditions, including coeliac disease, Type 1 diabetes and inflammatory bowel diseases, as well as many other inflammatory diseases. Notable stimulants to zonulin production are the bacteria E. coli and Salmonella, and the gluten product gliadin (implicated of course in coeliac diasese but likely to be a feature in other zonulin-assocated illnesses).
The diagram below shows how our understanding of the connections between the microbiome, gut wall and autoimmune disease has developed. Check on the link to read the review paper in full.
There are many forms of autoimmune disease, and in practice many more are never tidily categorised, Symptoms will depend on which tissues are primariy under attack (however what we have just learned is that the actual start of the disease may well be another event or location). As well as localised problems there are body-wide systemic versions of autoimmunity like lupus. We will look at the particular cases in the next three articles and at ME/CFS (Chronic Fatigue Syndrome) in our Plant Guide Gazette Fatigue Issue.
The following are the most common defined types of autoimmunity.
Addison’s disease. where the adrenal cortex is attacked and less steroid hormones are produced, with fatigue, patchy skin as signs of deeper problems in metabolsm and fluid balance.
Alopoecia areata attacks hair follicles leading to bare patches or a total loss of hair.
Ankylosing spondylitis (AS). See this post..
Coeliac disease. See here
Crohn’ disease. See this post
Graves disease stimulates the thyroid gland to make too much hormone – ‘hyperthyroidism’- with weight loss, anxiety, shaking, and maybe bulging eyes.
Guillain-Barré syndrome attacks the nerve network with tingly and weak arms and loss of sensation of heat or pain.
Hashimoto’s disease damages the thyroid to make too little hormone – ‘hypothyroidism – with weight gain, puffiness, sensitivity to cold, hair loss and fatigue.
Lupus (systemic lupus erythematosus) affects many parts of your body at the same time, with joint pain, sensitivity to light, rosacea face rash, kidney problems, and fatigue.
Multiple sclerosis (MS) targets the fatty myelin sheath that surrounds and protects nerve fibres. leading to pain, problems with movement and balance, and weakness.
Myasthenia gravis attacks the junctions between nerves and muscles leading to weakness and reduced control of movements particularly in eyes face and mouth.
Psoriasis. See this post. Some people with psoriasis have psoriatic arthritis, see here.
Rheumatoid arthritis (RA) see this post.
Type 1 diabetes targets the cells in the pancreas that make insulin.
Ulcerative colitis. See this post.
For a deeper dive into autoimmunity check out these recent review papers



Recent Comments